BVS ANALYSIS OF LFP WITH XRD
Introduction
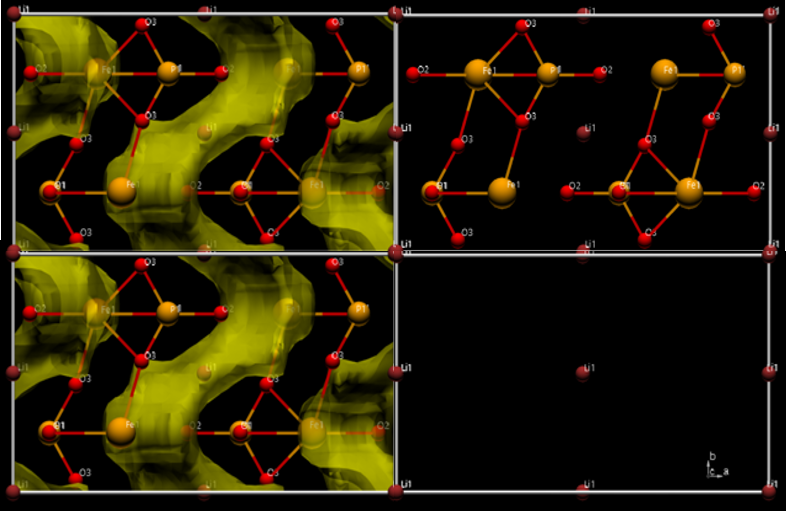
The valence of a positive ion can be evaluated by the BVS (Bond Valence Sum) method utilizing interatomic distances returned by Rietveld refinement. From the valance evaluation of Lithium Iron Phosphate (LFP), the valance of Li was calculated as 1+ and Fe was calculated as 2+. In addition, the diffusion path of the Li-ion (displayed in yellow) can be shown by simulating places where Li-ions tend to become monovalent in the crystal structure of LFP. The BVS method is an effective tool for material design and research into Li battery materials, allowing estimation of the diffusion paths for Li-ions.
Structure analytical result of Lithium Iron Phosphate
LFP (LiFePO4)
|
Element |
x |
y |
z |
Valence |
Bond Valence Sum |
|
Li1(Li) |
0.000000 |
0.000000 |
0.000000 |
1 |
0.977313897861123 |
|
Fe1(Fe) |
0.28231(9) |
0.250000 |
0.9744(3) |
2 |
1.90827098596528 |
|
P1(P) |
0.09511(17) |
0.250000 |
0.4188(4) |
0 |
0 |
|
O1(O) |
0.0948(4) |
0.250000 |
0.7422(8) |
0 |
0 |
|
O2(O) |
0.4563(4) |
0.250000 |
0.20847(7) |
0 |
0 |
|
O3(O) |
0.1638(3) |
0.0495(5) |
0.2848(5) |
0 |
0 |
BVS of Li is 0.98
≒ Univalent
BVS of Fe was 1.91
≓ Divalent